- Home
- Resources
- News
- Biogenix Labs collaborates with ADQCC to provide Proficiency Testing for COVID-19 PCR
Biogenix Labs collaborates with ADQCC to provide Proficiency Testing for COVID-19 PCR

Biogenix Labs collaborates with ADQCC to provide Proficiency Testing for COVID-19 PCR
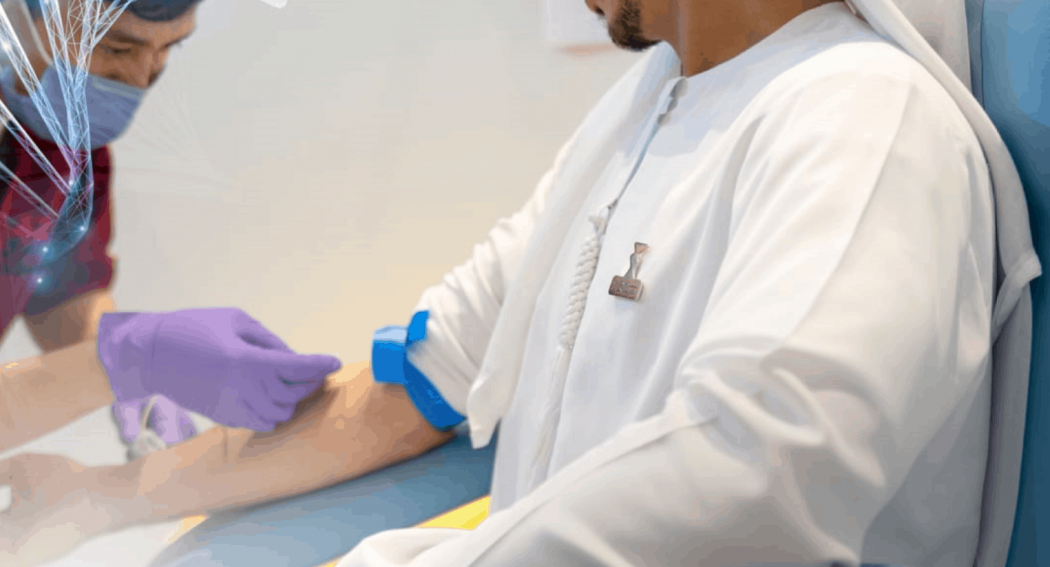
Biogenix Labs, a G42 Healthcare company, in collaboration with the Abu Dhabi Quality and Conformity Council (ADQCC), has launched the first Proficiency Testing (PT) program for COVID-19 external testing procedures, reaching out to laboratories across the UAE.
ADQCC was established in 2009 to establish and implement product safety, legal metrology and conformity schemes that inspire consumer confidence. The PT program is aimed at raising the standard of quality assurance for COVID-19 testing in the country, thus guaranteeing the accuracy and efficiency of diagnostic methods.
The PT process involved COVID-19 RT-PCR samples prepared by Biogenix Labs and was sent to over 15 government and private labs across the UAE. The results were then evaluated by ADQCC and sent back to the labs to check their quality standards. The initial survey samples were tested on 5 different platforms, including LamPORE which combines loop-mediated isothermal amplification with nanopore sequencing.
A market leader in the field, Biogenix Labs utilizes many platforms for COVID-19 molecular testing including RT-PCR diagnostic methods, isothermal amplification methods and LamPORE sequencing method. The lab conducts tens of thousands RT-PCR tests per day and has to date processed more than 600,000 samples since commencing services.
Ashish Koshy, CEO of G42 Healthcare, thanked ADQCC for its trust and said, “We are very pleased to collaborate with ADQCC and become the first laboratory in the UAE privileged to provide the COVID-19 PT program. We are ready to step up to the challenge of conducting peer reviews and are honored to set the benchmark in accurate and reliable testing. We will work together with UAE labs to troubleshoot any deficiencies discovered and raise testing standards to ensure that a high degree of safety, accuracy and efficiency is adopted for population-scale detection and diagnosis across the UAE.”
Biogenix Labs, part of G42 Healthcare, upholds the health of the community by maintaining the highest standards of quality and efficiency. It was also the first lab to achieve ISO15189 accreditation for COVID RT PCR and COVID antibodies (IgG & IgM) from the Emirates National Accreditation System (ENAS), the federal accreditation body of the UAE, and is compliant with Department of Health, Abu Dhabi (DOH) protocols and the international standards. This assures a high degree of confidence and reliability in the test results, a critical element in fighting the COVID-19 pandemic.
Located in the sustainable city of Masdar, Biogenix Labs operates to Biosafety Level 3 standards and is changing the landscape and meaning of reference laboratories with the fastest turnaround time, quality testing procedures and efficient processes. Its’ state-of-the-art equipment and processes include fully automated extractors, real-time PCR, liquid handlers, immunoassay, chemistry and hematology analyzers.