G42 Healthcare COVID-19 vaccine trials expand to Egypt

G42 Healthcare COVID-19 vaccine trials expand to Egypt
- Egyptian Ministry of Health to manage vaccine trials based on trial protocols developed in the UAE
- 6,000 volunteers set to participate in the trials
- Trials go Pan-Arab, notching another first, with volunteers now participating across four countries – UAE, Bahrain, Jordan and Egypt
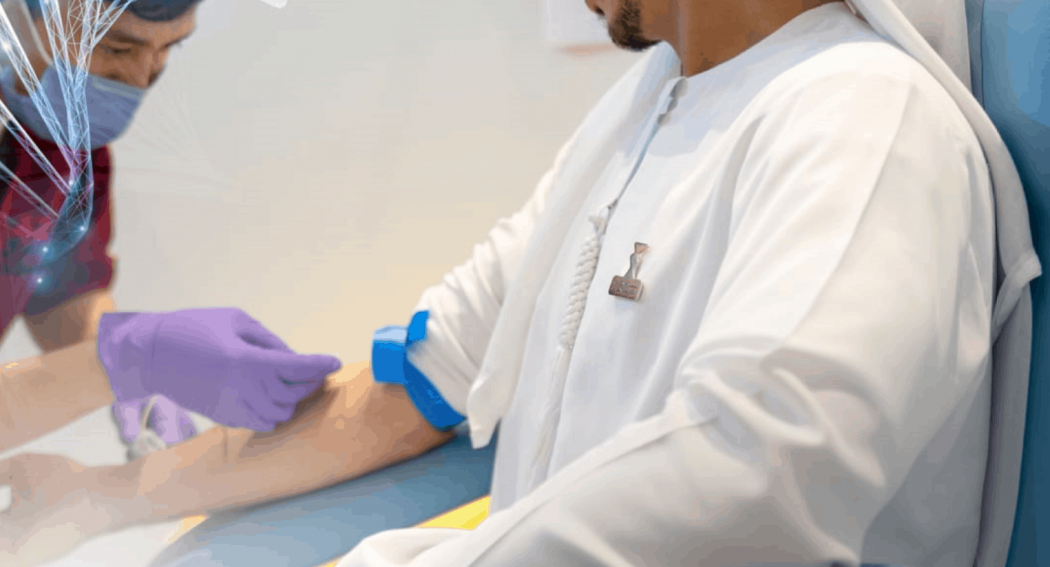
As part of the ongoing efforts to expand the reach of the Phase III clinical trials of an inactivated COVID-19 vaccine, a new centre for the 4Humanity programme was launched today in Egypt, making it the fourth Arab country to participate in these trials following the UAE, Bahrain and Jordan. The extension follows the successful launch of the trials just under two months ago in the UAE by G42 Healthcare and Sinopharm CNBG.
Volunteer numbers for the ongoing trials are set for a significant boost as a new centre opens at the Vacsera premises in Giza in the Greater Cairo area. It will screen and vaccinate volunteers who have registered on the Egyptian Ministry of Health and Population (MOHP) website (www.Covactrial.mohp.gov).
The trial process is being managed by the Egyptian Ministry of Health and Population with the support of UAE based G42 Healthcare and Sinopharm CNBG. The new centre is open to individual volunteers aged from 18 and above living in Egypt.
The Giza centre ensures that the 4Humanity trials are the first Pan-Arab initiative of its kind with four Arab countries now involved in the trials including the UAE, Bahrain, Jordan and Egypt. Recently the world’s first Phase III COVID-19 inactivated vaccine trials announced 31,000 vaccinated volunteers from 125 nationalities in the UAE alone, setting new benchmarks with these vaccinated volunteer numbers achieved in six weeks.
As in the other three countries, the trials in Egypt will follow the already established clinical protocols by the 4Humanity programme and the results will form part of the overall analysis and assessment of the trials.
Commenting on the start of the trials, Dr. Hala Zayed, Egyptian Minister of Health and Population, said: “The COVID-19 pandemic has had a major impact on every part of Egyptian society and we are delighted to have the opportunity to play our role in this vitally important research that can help find a successful vaccine to combat the biggest health crisis of our lifetime. Our participation in these clinical trials is a testament to Egypt’s leading role in the areas of science and research and reinforces our commitment to being part of the international efforts to find an effective vaccine for the global COVID-19 pandemic.
“We have been enormously impressed by the rigour and professionalism shown by G42 Healthcare and the 4Humanity trial protocols; its successful recruitment of volunteers to date and the use of its technological capabilities to speed up the analysis and findings. I am confident that we will see thousands of public-spirited Egyptians coming forward to play their part and participate in this important research.”
G42 Healthcare CEO Ashish Koshy added: “This latest international extension for the trials will give volunteer numbers another significant boost as we start the programme in North Africa, and the Arab world’s most populous nation and one of the oldest civilisations in the world that has been fighting plagues and pandemics through ancient history.
“It will play an important role in continuing to build scale and diversity and ensuring the number of volunteers the 4Humanity trials secure is a globally significant benchmark. We are looking forward to working closely with our partners at the Ministry of Health and Population in Egypt to ensure a successful next step in our ongoing trials.”
The trials began in Abu Dhabi on 16th July and are being managed by G42 Healthcare in partnership with the Department of Health – Abu Dhabi, the UAE Ministry of Health and Prevention (MOHAP) and Abu Dhabi Health Services Company (SEHA). They are being conducted following the international guidelines stipulated by the World Health Organization (WHO) and the United States Food & Drug Administration (USFDA).
The Phase III clinical trials follow the earlier success of the Phase I and Phase II trials conducted by Sinopharm in China, which resulted in 100% of the volunteers generating antibodies to SARS-CoV-2, the virus that causes COVID-19, after receiving two doses in 28 days.